Good to Know Series - 07 – Natural Graphite – Part 2: Crystallographic and Chemical Structure
To understand how certain properties of graphite vary, it’s helpful to first look at its crystallographic and chemical structure.
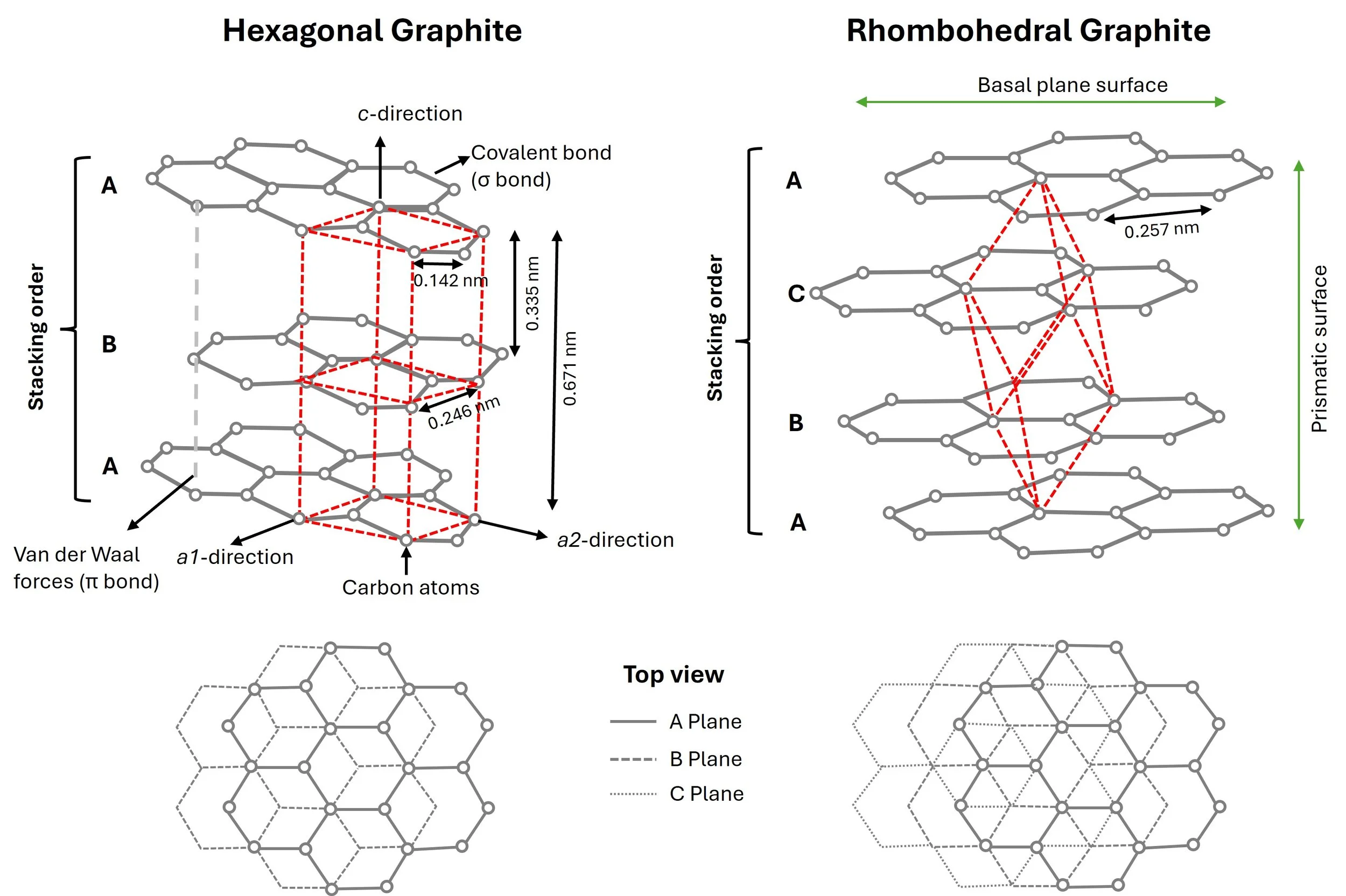
Graphite consists of parallel layers—also known as graphene layers—that are weakly bound together by van der Waals forces (associated with π bonds). Depending on the stacking sequence, graphite can exist in two structural forms:
Hexagonal graphite (also known as Bernal stacking, AB sequence), described by Bernal (1924), and
Rhombohedral graphite (ABC stacking), described by Lipson and Stokes (1942).
These two phases can coexist in the same sample (Kwiecińska and Kajzar, 1975). The figure below illustrates both structures. As Bernal (1924) described:
“The atoms of carbon in graphite lie in planes in which they form nets of hexagons. These nets are in successive planes, superposed so that half the atoms in one net lie normally above half the atoms of the net beneath, while the other half lie normally above the centres of the hexagons of this net. Alternate nets lie atom for atom normally above each other.”
Crystallographic Details
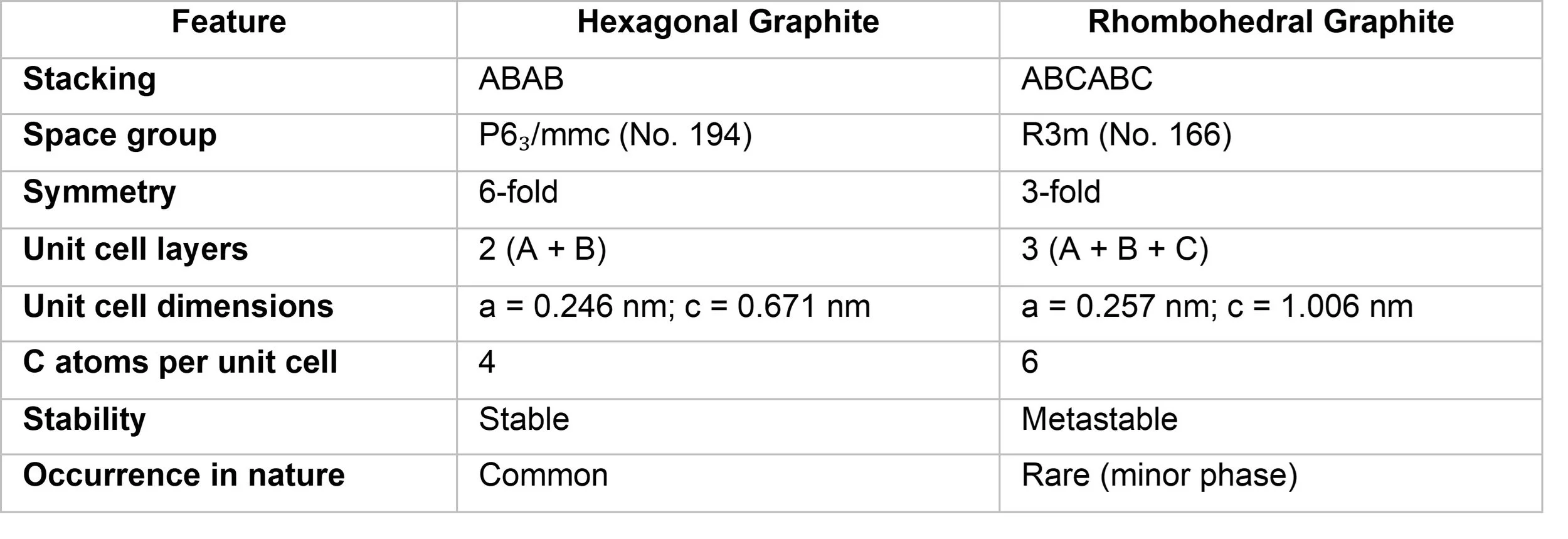
Hexagonal graphite belongs to the space group P6₃/mmc (dihexagonal dipyramidal crystal class), with unit cell dimensions of a = 0.246 nm and c = 0.671 nm. The interlayer spacing (distance between adjacent graphene layers) is approximately 0.335 nm.
Rhombohedral graphite is a metastable allotrope of graphite, described by space group R3m, with unit cell constants a = 0.257 nm and c = 1.006 nm. This form is not thermodynamically stable and typically reverts to hexagonal stacking when heated above 1350 °C.
Chemical Bonding and Hybridization
Carbon’s electron configuration is 1s² 2s² 2p², and in graphite it undergoes sp² hybridization:
The three sp² orbitals form σ (sigma) bonds with neighbouring carbon atoms in a flat, hexagonal 2D network. Within each layer, atoms are arranged with a nearest-neighbour distance of 0.142 nm.
The remaining p_z orbital extends above and below the plane, overlapping with adjacent orbitals to form π (pi) bonds. These π bonds create a delocalized electron cloud, giving graphite its high in-plane electrical conductivity and chemical stability.
Anisotropy in Graphite
Due to its bonding structure, graphite is highly anisotropic—its properties differ significantly depending on direction:
The in-plane (basal plane) is strong and conductive, thanks to the robust covalent bonding.
The out-of-plane (prismatic surface) is weak and easily cleaved, due to the much weaker van der Waals forces.
This anisotropy explains why graphite is useful as a lubricant and why it leaves marks when used in pencils.
Summary Table: Crystallographic Features
Defects in Real-World Graphite
While the ideal graphite structure is well understood, natural and synthetic graphite often contain defects that influence their properties. These include:
Stacking faults – irregularities in the AB or ABC sequence
Dislocations and vacancies – missing or misaligned atoms within layers
Intercalated species – foreign atoms or molecules inserted between graphene layers
Turbostratic disorder – misaligned or rotationally disordered layers
Such imperfections can affect properties like thermal and electrical conductivity, mechanical strength, and reactivity, especially in industrial applications. Natural graphite tends to be more crystalline but can contain impurities, while synthetic graphite can be more controlled but may include engineered porosity or disorder, depending on how it’s produced.
Graphite and Graphene
Each graphene layer in graphite is essentially a single sheet of sp²-bonded carbon atoms arranged in a hexagonal lattice. Graphene is, therefore, the monolayer building block of graphite.
In graphite, multiple graphene layers stack in a specific sequence (AB or ABC), held together by van der Waals forces.
Graphene itself, first isolated in 2004 (Geim and Novoselov, 2007), exhibits extraordinary properties like ultrahigh electron mobility, mechanical strength, and transparency, which are somewhat masked in bulk graphite due to interlayer interactions.
Understanding this relationship helps explain why exfoliated graphite, expanded graphite, and graphene-based materials are so important in emerging technologies like flexible electronics, composites, and energy storage.
References
Bernal, J.D., 1924. The structure of graphite. Proceedings of the Royal Society London A 106, 749–773.
Dana, J. D., Palache, C., Berman, H., & Frondel, C.,1944. The System of Mineralogy: Volume I. John Wiley & Sons.
Geim, A.K., Novoselov, K.S., 2007. The rise of graphene. Nature Materials 6, 183-191.
Kwiecińska, B., Kajzar, F., 1975. The rhombohedral structure contribution in natural graphites determined by neutron diffraction technique. Mineralogia Polonica 6, 25–32.
Lipson, H., Stokes, A.R., 1942. The structure of graphite. Proceedings of the Royal Society London A 181, 101–105.